
Eric Larsen, the Business Development Lead at The Clarient Group, recently attended the 2025 ISPE (International Society for Pharmaceutical Engineering) Annual Meeting in Charlotte to better understand how life sciences organizations are navigating digital transformation. The event, centered on ISPE’s Pharma 4.0 framework, made one theme unmistakably clear: while the industry’s digital ambitions are bold, its current operational posture remains years behind.
Across sessions, case studies, and vendor discussions, the most significant barriers were not technical limitations but organizational readiness, data governance maturity, and change management. The conference focused heavily on how the industry can bridge this gap through clearer digital strategies, better alignment across stakeholders, and more standardized approaches to data.
The State of the Industry: Big Vision, Long Runway
A consistent message emerged: as leaders champion AI, predictive models, and automation, day-to-day operations still depend heavily on aging systems, fragmented data, and siloed teams. Many pharmaceutical organizations are 10–30 years away from achieving the fully automated, data-driven facilities they aspire to.
Notably, the conversations pointed to two trends that mirror what we see in the smart building world:
- System integrators expanding into digital strategy. Many integrators now market “digital integration” or MSI-like services, but often through a systems-first rather than data- or business-first lens.
- Software consolidation is driving owner frustration. Rising licensing and SaaS costs, highlighted by examples such as Schneider Electric’s acquisition of Aveva (developer of the OSI Pi historian), are pushing owners to explore alternatives.
These pressures are opening space for more holistic, vendor-agnostic digital strategies—an area where clients increasingly seek support.
Pharma 4.0: ISPE’s Framework for a Digital Future
Pharma 4.0 is ISPE’s structured model for applying Industry 4.0 principles in pharmaceutical settings. As biologics, cell therapies, and vaccines accelerate R&D timelines, speed-to-market has become the industry’s primary performance metric. Pharma 4.0 aims to remove the systemic barriers that slow progress.
Key challenges the framework addresses include:
- High implementation costs and reliance on legacy systems
- Growing cybersecurity risks
- Workforce shortages and skills gaps
- Increasing sustainability expectations
Pharma 4.0 is not positioned as an IT initiative, but as a cross-organizational transformation model that requires aligned culture, processes, and data infrastructure. Its foundation is data integrity – enabled by disciplined quality systems, standardized processes, and strong governance.
Spotlight: AstraZeneca’s Pharma 4.0 Approach
One of the most compelling sessions came from AstraZeneca’s Philip Ceary, who shared lessons from the company’s new Dublin facility, designed from inception around Pharma 4.0 principles.
Key Use Cases
- Predictive maintenance for critical equipment
- Process simulation and digital twins
- Real-time operational performance monitoring
Key Takeaways
- Early, cross-disciplinary engagement was essential to aligning on use cases and data requirements.
- The project intentionally broke down IT/OT barriers, creating a seamless enterprise data environment.
- Standardized, “democratized” data access empowered operators, engineers, and contractors alike.
- For retrofits, Ceary emphasized: “Start by defining what you want to achieve.” Technology is secondary to clarity of purpose.
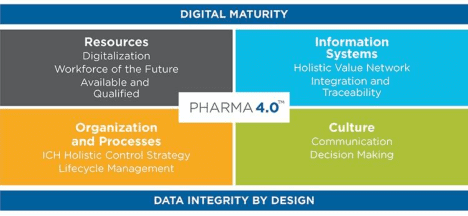
Spotlight: Benchmarking Digital Maturity
A separate session explored how organizations can assess their readiness for Pharma 4.0 using three leading maturity models:
- BioPhorum DPMM: Focused on biopharma, process development, and cybersecurity
- ISPE’s Pharma 4.0 Assessment: Creates a visual heat map across Resources, Organization & Processes, Information Systems, and Culture
- SIRI (Smart Industry Readiness Index): A World Economic Forum-supported, industry-agnostic model
The most significant barrier is not the assessment method – it’s getting 8–15 stakeholders aligned and in the same room for the 1–2 days required to complete an honest evaluation. This echoes the organizational challenges repeatedly highlighted throughout the conference.
Conclusion: The Industry Needs Strategic Guidance, Not More Technology
The 2025 ISPE Annual Meeting reinforced that digital transformation in life sciences is no longer optional. Pharma 4.0 is emerging as the industry’s leading framework, but the gap between the model and current reality is wide.
The challenges ahead are fundamentally strategic and cultural, not technical. Organizations need partners who can:
- Align stakeholders around a unified digital vision
- Establish data governance and integration requirements early
- Navigate change management across operational, IT, QA/QC, and engineering teams
- Remain technology-agnostic while focusing on outcomes
These needs align directly with The Clarient Group’s core strengths: digital strategy, integration planning, and cross-disciplinary coordination that position organizations for long-term, scalable transformation.